Detection method of fluoride ion in aqueous solution
Determination of fluoride in water (fluoride ion selective electrode method)
1. Principle The fluoride ion selective electrode and external reference electrode (such as calomel electrode) are immersed in the fluorine-containing solution to be tested to form a primary battery. The electromotive force of the primary battery has a linear relationship with the logarithm of the fluoride ion activity, so by measuring the electromotive force of the primary battery composed of the electrode and the known F-concentration solution and the electromotive force of the primary battery composed of the electrode and the F-concentration solution to be measured Calculate the F-concentration in the water sample to be measured. Commonly used quantitative methods are standard curve method and standard addition method.
Severely polluted domestic sewage and industrial wastewater, as well as water samples containing fluoroborate, must be distilled.
2. Instruments
1. PF-1 fluoride ion selective electrode. Can be replaced with PF-1C composite fluoride ion electrode
2. 217 type 232 type saturated calomel electrode or 218 silver-silver chloride electrode.
3. PXS-215A ion activity meter or PHS-3C acidity meter, accurate to 0.1mV.
4. ES-3072 magnetic stirrer, stirrer wrapped in polyethylene or Teflon.
5. Polyethylene cup: 100mL, 150mL.
6. Other commonly used laboratory equipment.
3. The water used for the reagent is deionized water or fluorine-free distilled water.
1. Fluoride standard stock solution: Weigh 0.2210g of standard sodium fluoride (NaF) (dry in advance at 105-110 ℃ for 2h, or at 500-650 ℃ for about 40min, cool), dissolve with water and transfer to 1000mL volumetric flask Medium, dilute to the mark and shake well. Store in polyethylene bottles. This solution contains 100ug of fluoride ion per ml.
2. Fluoride standard solution: Pipette 10.00mL of sodium fluoride standard stock solution with a graduated pipette, pour into a 100mL volumetric flask, dilute to the mark, and shake well. This solution contains 10ug of fluoride ion per ml.
3. Sodium acetate solution: Weigh 15g of sodium acetate (CH3COONa) in water and dilute to 100mL.
4. Total ionic strength adjustment buffer solution (TISAB): Weigh 58.8g sodium citrate dihydrate and 85g sodium nitrate, add water to dissolve, adjust the pH to 5-6 with hydrochloric acid, transfer to a 1000mL volumetric flask, dilute to the mark, shake well .
5.2 mol / L hydrochloric acid solution.
Fourth, the measurement steps ----- fluoride ion detection method in aqueous solution
1. Instrument preparation and operation According to the instructions of the measuring instrument and electrode used, first connect the circuit, put each switch in the "off" position, turn on the power switch, preheat for 15min, and then operate according to the instructions. Before the measurement, the test solution should reach room temperature and be consistent with the temperature of the standard solution (temperature difference should not exceed ± 1 ℃).
2. Standard curve drawing: Draw 1.00, 3.00, 5.00, 10.00, 20.00mL fluoride standard solution with non-dividing pipette, put them into 5 50mL volumetric flasks respectively, add 10mL total ionic strength adjustment buffer solution, dilute with water to the mark, Shake well. Move them into 100mL polyethylene cups respectively, put a plastic stirrer in each order, insert the electrodes in order from low to high concentration, continuously stir the solution, and read the steady-state potential value (E) under stirring. Before each measurement, the electrode must be rinsed with water and absorbed with filter paper. Draw the E-lgcF-standard curve on semi-logarithmic graph paper, the concentration is marked on the logarithmic grid, and the lowest concentration is marked on the starting line of the abscissa.
3. Water sample determination: draw a proper amount of water sample with a graduated pipette, place it in a 50mL volumetric flask, adjust to near neutrality with sodium acetate or hydrochloric acid solution, add 10mL of total ionic strength adjustment buffer solution, dilute with water to the mark, and shake well. Move it into a 100mL polyethylene cup, put a plastic stir bar, insert the electrode, and continuously stir the solution. After the potential is stabilized, read the potential value (EX) while continuing to stir. Before each measurement, the electrode must be thoroughly washed with water and absorbed with filter paper. According to the measured millivolts, the fluoride content can be found from the standard curve.
4. Blank experiment: replace the water sample with distilled water, and measure according to the conditions and steps of the sample.
When the composition of the water sample is complex or the composition is unknown, the standard addition method should be used once in order to reduce the influence of the matrix. The operation is as follows: first measure the potential value (E1) of the test solution according to step 2, then add a certain amount of fluoride standard solution (similar to the fluorine content in the test solution) to the test solution, and read the steady state under constant stirring Potential value (E2).
V. Calculation
1. Standard curve method: The fluoride content (mg / L) in the water sample can be calculated according to the concentration and dilution factor of the diluted water sample from the standard curve.
2. , Standard Joining Method
cx = (cs · VS) / (Vx + Vs) * (10 * ΔE / s-Vx / (Vx + Vs))-1
In the formula: cx ——— Fluoride (F-) concentration in water sample (mg / L);
Vx ——— Water sample volume (mL);
cs ———— F—the concentration of standard solution (mg / L);
VS ——— Volume of added F—standard solution (mL);
ΔE——equal to E1–E2 (for anion selective electrode), where E1 is the measured potential value (mV) of the water sample solution, and E2 is the measured potential value (mV) after the standard solution is added to the test solution;
S——The measured slope of the fluoride ion selective electrode.
If VS << VX, the above formula can be simplified to:
cx = cx · VS (10 △ E / S-1) -1 / Vx
Precautions
1. After the electrode is used, rinse it thoroughly with water, and absorb the water with filter paper, and put it in the air or in a dilute fluoride standard solution. If it will not be used for a short period of time, it should be washed, absorbed moisture, and put on a protective cap to protect the sensitive parts of the electrode. The electrode should still be washed before use, and absorb moisture.
2. If the fluoride content in the test solution is low, the blank test value should be deducted from the measured value.
3. Do not touch the sensitive membrane of the electrode by hand; if the surface of the electrode membrane is contaminated with organic matter, etc., it must be cleaned before use.
4. The concentration of the standard solution (cS) added by the one-time standard addition method should be 10-100 times higher than the concentration of the test solution (cX), and the volume added is 1 / 10-1 / 100 of the test solution, so that the TISAB concentration of the system does not change Big.
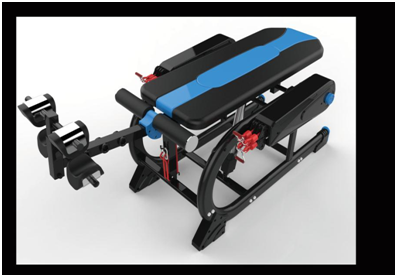
The Electric Inversion Table is a good quality extreme
performance Inversion Table . It is a keep fit and entertainment inversion
table . It can 180 degree to rotate and only need to press the button . The Max
bear weight is 150KGS and all the parts are big and stronger . It is also a multifunctional
inversion table. It can handstand and pull up and down . So this inversion
table price is a bit high . Because it is has much function ,also the quality
is better ,so still many peoples like it very much . It is hot sell and popular
welcome in the whole USA sports markets .
|
XJ-TFE-04 |
|||||||
|
SPECIFICATION |
|||||||
|
G.W |
38KGS |
N.W |
35KGS |
Max Weight |
150KGS |
Meas |
134*47*24 CM |
|
Max Height |
198CM |
Size |
145*47*62CM |
QTY |
1PC/CN |
||
|
Ctn |
185PCS/20`FT |
380PCS/40`GP |
445PCS /40`HQ |
|
|||
Electric Inversion Table
Electric Inversion Table,Inversion Table With Safety Belt,Extreme Performance Inversion Table,Gym Electric Inversion Table
ZHEJIANG MEIER FITNESS EQUIPMENT CO., LTD , http://www.chinameier.com